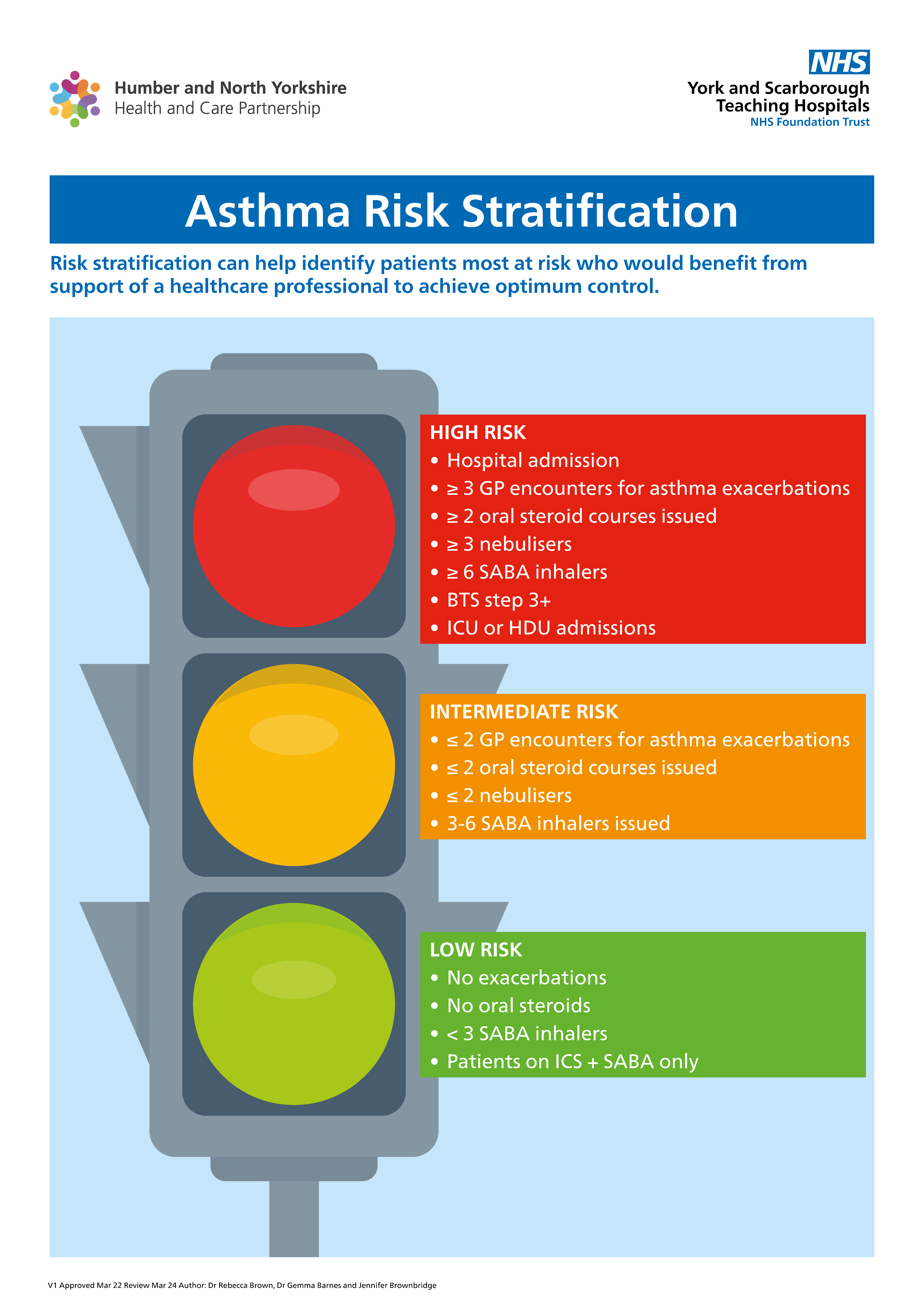
Asthma in children (chronic)
Definition/Description
A long-term condition affecting both children and adults. Inflammation and narrowing of the small airways in the lungs cause asthma symptoms, such as cough, wheeze, shortness of breath and chest tightness
- Most common chronic disease among children
- Acute wheeze is one of the most common reasons for emergency department attendance and hospital admission in children
- Triggers can include viral infections, dust, smoke, fumes, changes in the weather, grass and tree pollen, animal fur and feathers, strong soap and perfume
- Symptoms are intermittent and often worse at night or during exercise
- It is more likely in people who have other allergic conditions, such as eczema and rhinitis
- Early life factors can increase risk of asthma such as low-birth weight, prematurity, tobacco exposure and air pollution
- Overweight or obese individuals are at a greater risk of asthma
- Each year, there are still a small proportion of avoidable deaths in children and young people resulting from asthma
Red Flag Symptoms
None
Guidelines on Management
Differential Diagnoses
It is important to differentiate between viral induced wheeze, other causes of wheeze and asthma.
- Pneumonia: pyrexia >38.5°C, productive cough, asymmetry on auscultation
- Epiglottitis: dysphagia, drooling
- Croup: inspiratory stridor
- Hyperventilation: breathlessness with light headedness and peripheral tingling
- Foreign body: localized wheeze and reduced air entry
- GORD: excessive vomiting
- Anaphylaxis
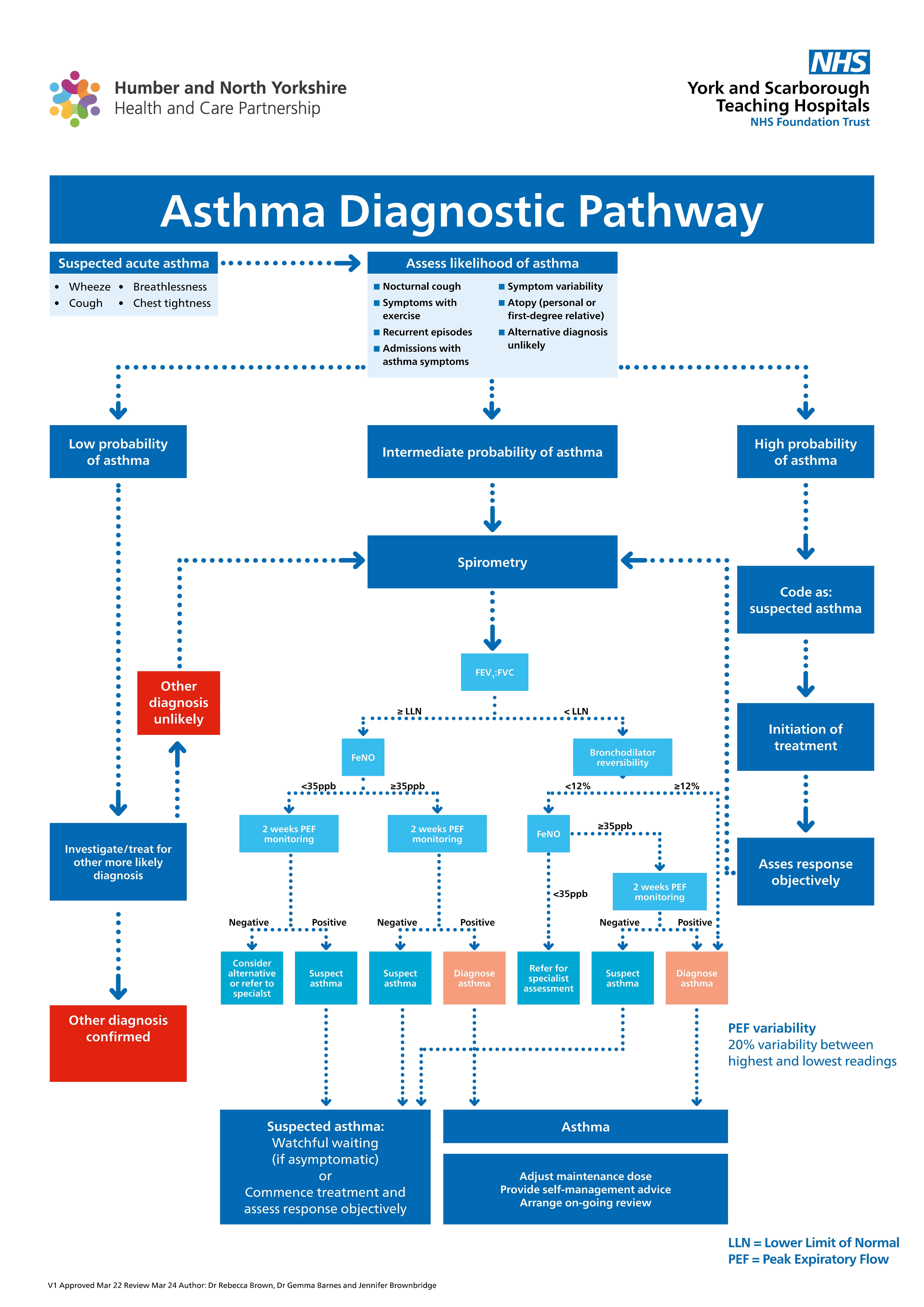
Assessment
Diagnosis is based on the recognition of a characteristic pattern of respiratory symptoms, signs and test results and the absence of any alternative explanation for these.
Making the diagnosis
Asthma should be confirmed when the child is able to undergo objective testing, usually over the age of 5 years
Supportive Investigations |
|
Spirometry |
<FEV1/FVC ration <70% |
Bronchodilator reversibility |
Improvement in FEV1 of ≥ 12% |
Peak flow variability |
Peak expiratory flow variability >20% over a 2-4 week period |
Exhaled nitric oxide ‘FeNO’ |
FeNO >35ppb |
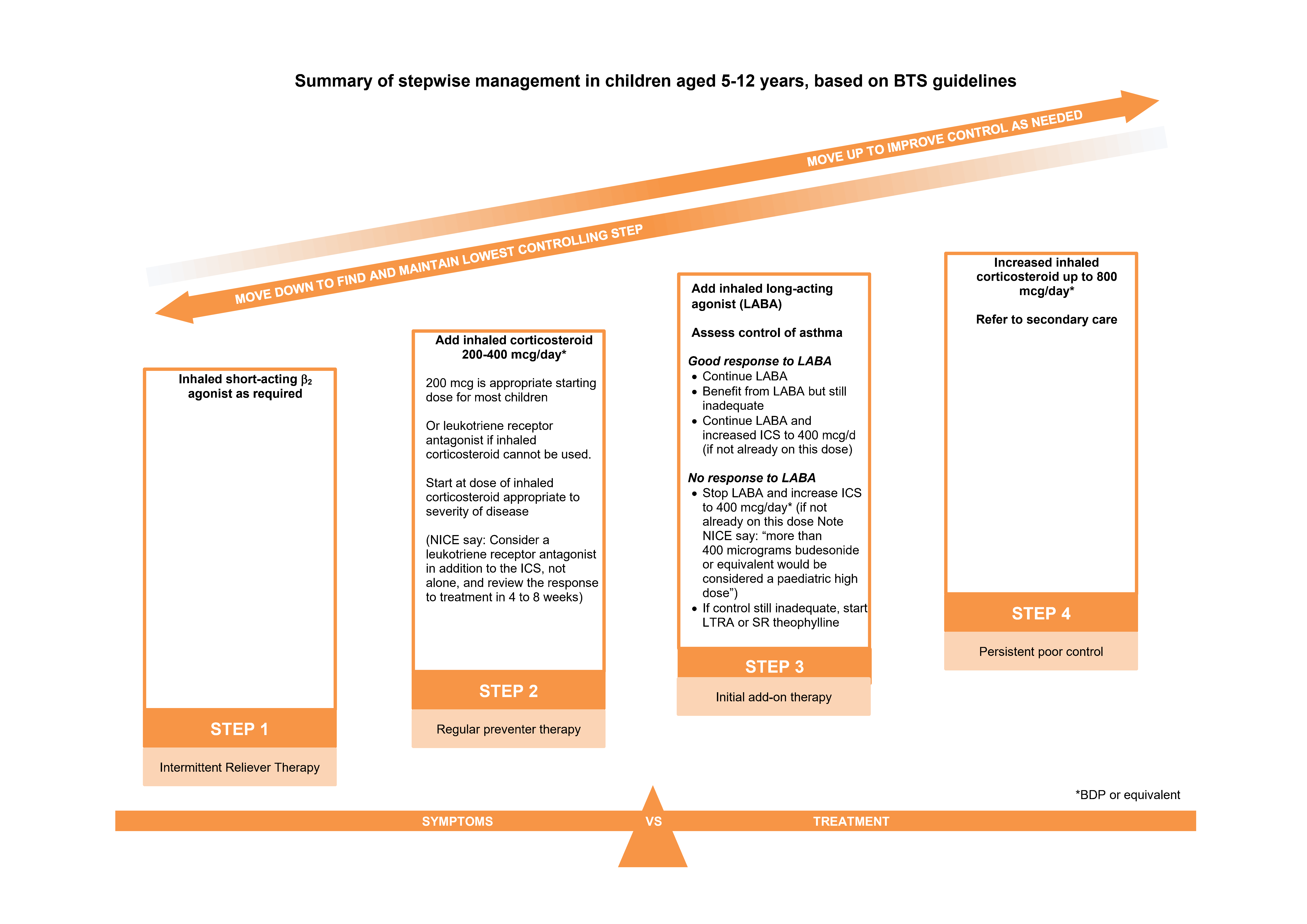
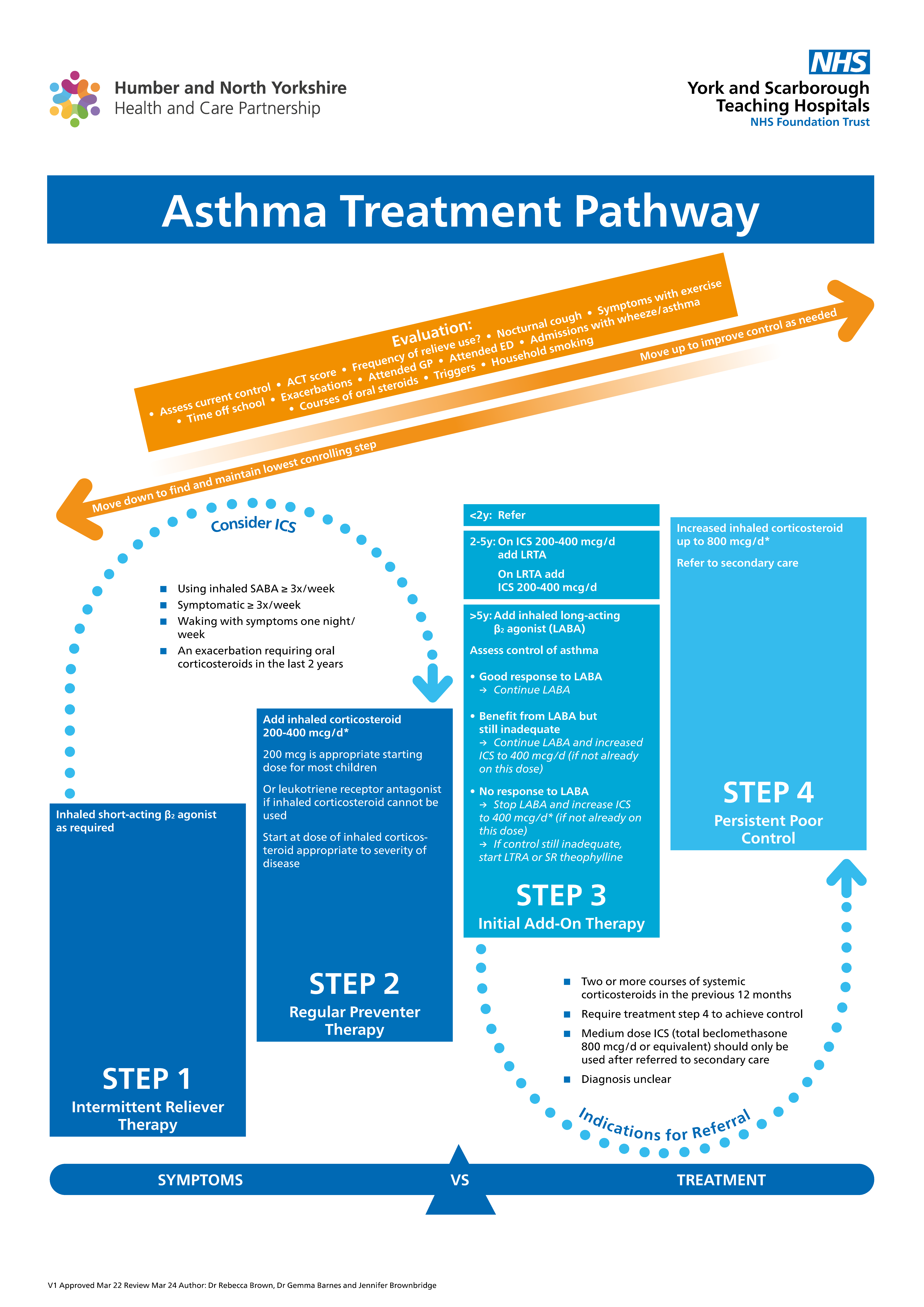
Chronic Management
Key Principles
- Start treatment at the step most appropriate to initial symptom severity
- Check adherence and inhaler technique and reconsider diagnosis if poor response
- Aim is to achieve early control and maintain at lower possible dose
- Step treatment up and down accordingly
- Use of 6 short acting bronchodilator devices in 12 months should be considered for review
- Those using ≥ 12 in 12 months should be invited for an urgent review, this is associated with an increased risk of asthma death
- Provide patients with a personalised patient action plain
- Weight-loss interventions can be considered for overweight and obese children with asthma to improve control
Inhalation Device
- For children >5y: pMDI + spacer is first choice inhalation device for ICS.
- For SABA, consideration should be given to a wider range of devices. However, children should always have access to Salbutamol pMDI and spacer, in the event of a significant asthma attack.
- >5y use mouthpiece instead of mask (providing technique is good)
- Patients should be given adequate training in the use of the inhalation device
- Clean spacers once a month with mild detergent and allow to air dry. Replace every 6- 12months.
- Consideration should be made to the carbon footprint of inhalation devices. DPIs are less harmful than pMDI and should be used when clinically appropriate.
In brief to achieve optimum lung deposition, the inspiratory effort should be:
- pMDI: SLOW AND STEADY, using a spacer device
- DPI: QUICK AND DEEP
Intermittent Reliever Therapy
- Children should be prescribed a short-acting bronchodilator to relieve symptoms
- For those with infrequent short-lived symptoms, occasional use of reliever may be all that’s required
Short acting beta2 agonists (SABAs)
Salbutamol
- Child, by aerosol inhalation: 100–200 micrograms (1–2 puffs); for persistent symptoms as per personalised asthma action plan.
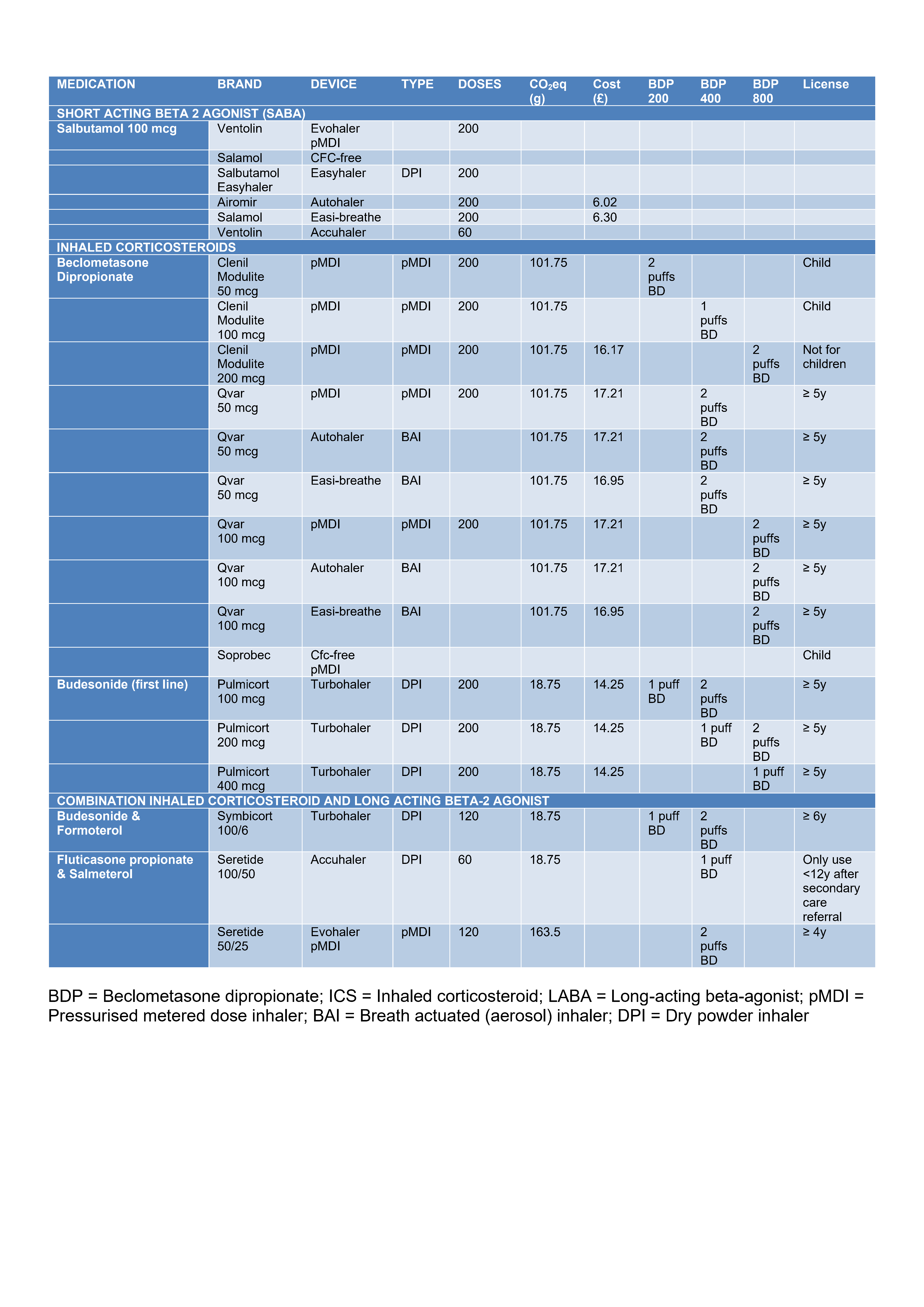
(see Image B)
Regular Preventer Therapy
Inhaled Corticosteroids
- Trial any change involving an inhaled corticosteroid for at least 6 weeks
- ICS are the most effective preventer drug for achieving overall treatment goals
- In mild to moderate asthma in children, a usual starting dose total BDP 200 micrograms /d or equivalent
IMPORTANT
Consider ICS with any of the following
- Using inhaled SABA ≥ 3x/week
- Symptomatic ≥ 3x/week
- Waking with symptoms one night/week
- An exacerbation requiring oral corticosteroids in the last 2 years
Beclometasone Dipropionate
Clenil Modulite: aerosol inhalation
50 mcg/dose inhaler and 100 mcg/dose inhaler; are licensed for use in children
200 mcg/dose inhaler and 250mcg/dose inhalers; are not licensed for use in children
- 5-11y: 100-200 micrograms twice daily
- >12y: 200-400 micrograms twice daily
Budesonide
Pulmicort Turbohaler (DPI): dry powder inhaler 100 mcg/dose, 200 mcg/dose, 400 mcg/dose
- 5-12y: 100-400 micrograms twice daily
- >12y: 100-800 micrograms twice daily
Other preparations less commonly used include Soprobec and Qvar. Qvar® has extra-fine particles, is more potent than traditional beclometasone dipropionate CFCcontaining inhalers and is approximately twice as potent as Clenil Modulite®.
Initial Add-on Therapy
- Not all brands and strengths of ICS/LABA inhalers are licensed for use under 18 years
- DO NOT prescribe LABA without ICS preventer treatment
<2y: consider referral to secondary care
2-5y: consider LTRA as initial add on therapy
5-12y: an inhaled LABA can be considered as initial add on therapy, or LTRA
Combination ICS/LABA
Inhalers Fluticasone propionate/salmeterol
Seretide 50 Evohaler (pMDI):
50/25 is only for use in children
• 4-17y: 2 puffs BD, reduced to 2 puffs once daily if control is maintained
Seretide 100 Accuhaler (DPI):
100/50: medium dose ICS should only be used in children 12y after referral to secondary care
- 4-17y: 1 puff BD, reduced to 1 puff once daily if control is maintained
Budesonide/formoterol
Symbicort Turbohaler:
100/6 is licensed for children aged 6 years and above
200/6 is licensed for children aged 12 years and above
- 6-17y: 1-2 puffs twice daily, reduced to 1 puff daily if control is maintained
- >12y: 200-400 micrograms twice daily
Leukotriene receptor antagonists (LTRAs)
Montelukast:
Consider if exercise induced asthma is a specific problem in a patient otherwise well controlled. Trial treatment for 4 weeks, if no benefit then discontinue. Risk of neuropsychiatric reactions such as speech impairment (stuttering) and obsessive-compulsive symptoms
- Tablets 10mg
- Chewable tablets 4mg, 5mg
- Granules 4mg
- 6m-5y: 4mg once daily in the evening
- 6-14y: 5mg once daily in the evening
Monitoring
- Conduct a routine clinical review annually
- Check inhaler technique and adherence – VERY IMPORTANT
- Assess current symptom control; symptoms, limitation on activity and use of rescue medication
- Impact on daily activities include sport, play and social life
- Asthma attacks
- Oral corticosteroid use
- Time off school
- Review personalised patient action plan
- Exposure to tobacco smoke
- Growth (height and weight) annually
Referral Criteria/Information
Indications for Referral
- Two or more courses of systemic corticosteroids in the previous 12 months
- Require treatment step 4 to achieve control
- Medium dose ICS (total beclomethasone 800 mcg/d or equivalent) should only be used after referred to secondary care
- Diagnosis unclear
Information to include in referral letter
- Exacerbation history: number of exacerbations in past 12 months
- Number of courses of oral steroids in past 12 months
- History of atopy: include any known triggers
- Current medication
- If possible a recent peak flow record with symptom diary over two weeks
Additional Resources & Reference
Patient information leaflets/ PDAs
Patient.info/chest-lungs/asthma-leaflet
Oxfordhealth.nhs.uk/Asthma-advice-for-children
Healthcare Professional Diagnostic Pathways
Healthcare Professional Treatment Pathways
Formulary for Inhaler Preparations Containing corticosteroids
Personalised Patient Action Plan
Child
Asthma.org.uk/children/my-asthma-plan-2021
Young Person
Asthma.org.uk/health-advice/resources/adults/asthma-action-plan
References
- National Institute for Clinical Excellent [NICE] (2020) Asthma: Diagnosis, monitoring and chronic asthma management [Viewed 16 Aug 2021]
- British Thoracic Society/Scottish Intercollegiate Guidelines Network 2019. British guidelines on management of asthma. [online]
- Royal College of Physicians of London, British Thoracic Society and British Lung Foundation. Why asthma skill kills: The national review of asthma deaths (NRAD). Confidential enquiry report. London (2015) [Viewed 16 Aug 2021] https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills
- National Institute for Clinical Excellent [NICE] (2021) Asthma – Clinical Knowledge Summaries. [Viewed 16 Aug 2021]
Appendix
Brand: All inhalers to be prescribed by brand especially combination inhalers
Spacer: Replace once a year
Combination inhalers: Recommended to ensure long action 2 agonist is not taken without inhaled corticosteroid
See image A below
Environmental impact
pMDI contain hydrofluorocarbon (HFC) propellants that are powerful greenhouse gases that can contribute to global warming.
Dry powder inhalers or soft mist inhalers are generally preferred locally, unless there is a specific clinical or dexterity reason that an individual requires a pMDI or BAI.
In young children, a pMDI and a spacer is the preferred method of delivery; a face mask is required until the child can breathe reproducibly using the spacer mouthpiece. A pMDI plus spacer is also recommended for patient of any age for the treatment of mild and moderate acute asthma attacks.
Inhalers should be disposed of in pharmaceutical waste bins and not in general waste. Spacer cannot currently be recycled
Associated Policies
Specialties
Places covered by
- Vale of York
Hospital Trusts
- York and Scarborough Teaching Hospitals