Post-menopausal bleeding (PMB) and unscheduled bleeding on HRT
Definition/Description
PMB is defined as an episode of vaginal bleeding 12 months or more after menstruation has stopped because of the menopause.
This pathway is for:
- All persons with a uterus who are postmenopausal experiencing PMB (Section 4.1)
- Women using HRT experiencing unscheduled bleeding (please see Section 4.2)
This pathway is not for:
- Heavy Menstrual Bleeding in pre-menopausal women
- Intermenstrual Bleeding in pre-menopausal women
PMB is a cardinal symptom of endometrial cancer and does require further investigation. Approximately 10% of women up to 60 years of age presenting and referred with post-menopausal bleeding have endometrial cancer, and this rises to 13% over the age of 60. For women on HRT presenting with PMB or unscheduled bleeding this risk falls to 1%.
Other causes of bleeding from the genital tract also need to be considered such as cervical/vaginal/vulval cancer, vaginal atrophy, and HRT.
Endometrial cancer can also present with unexplained vaginal discharge or haematuria.
Red Flag Symptoms
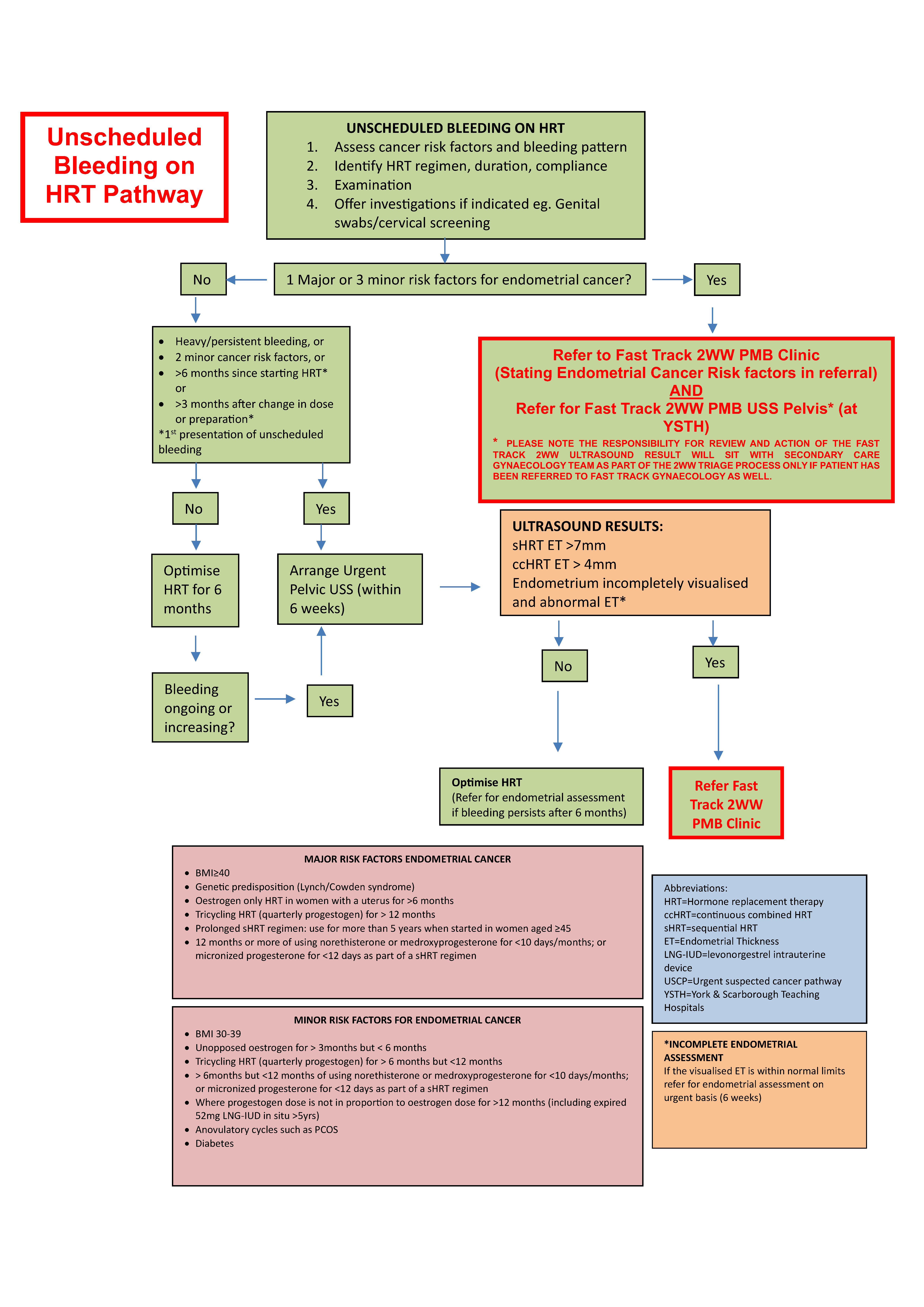
Risk factors for endometrial cancer:
|
Major Risk Factors |
Minor Risk Factors |
|
|
Guidelines on Management
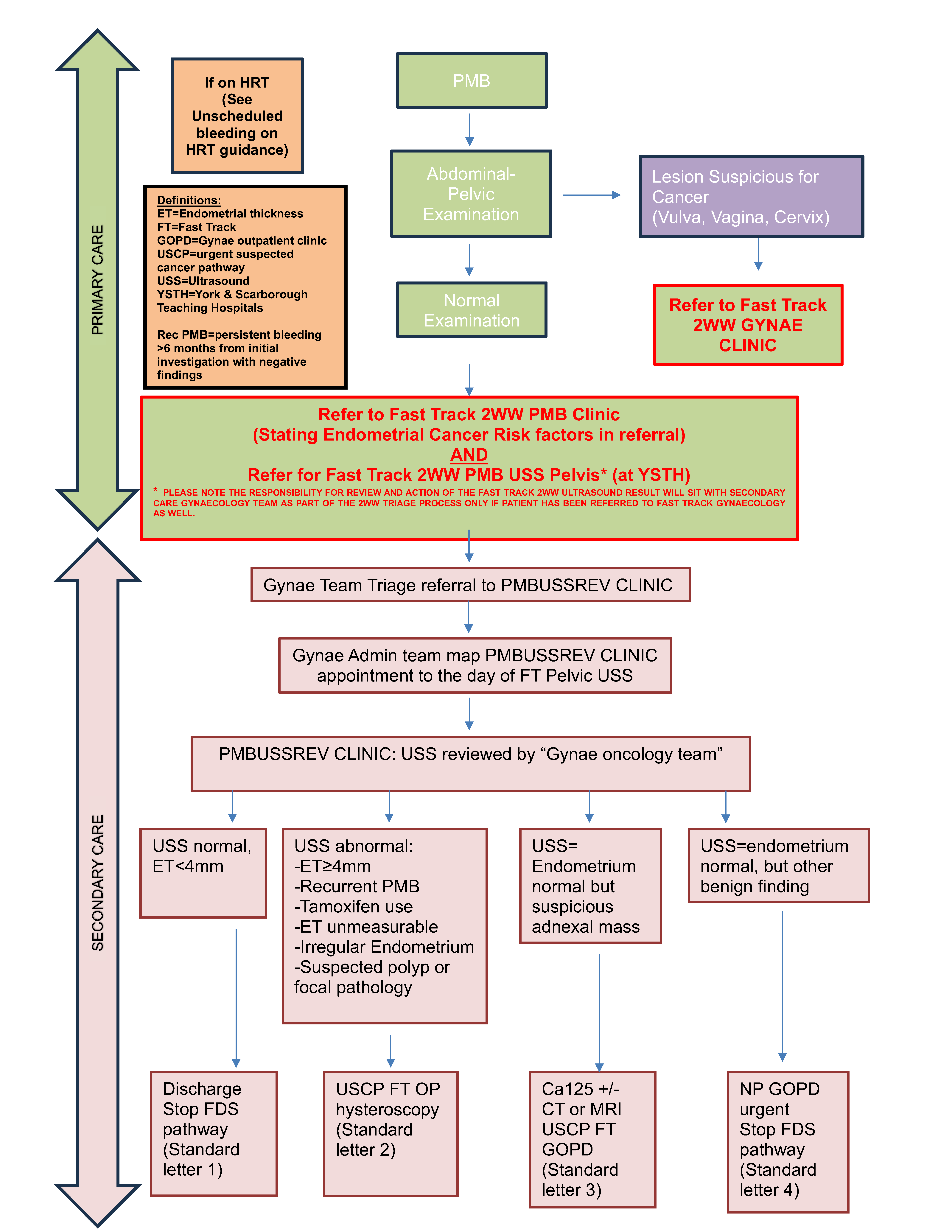
Management of PMB in Primary Care.
- Establish patient has PMB (see notes below regarding recurrent PMB)
- Take full history, perform gynaecological examination, genital swabs, and urinalysis
• It is essential to examine the lower genital tract to exclude vaginal/vulval/cervical cancer, if abnormalities are found please make a Fast Track/2WW referral using the referral proforma.
• Note any risk factors for endometrial cancer - If endometrial cancer is suspected:
• Make referral to Fast Track Gynaecology 2WW PMB (Urgent suspected Cancer Pathway)
AND
• Make referral/request for Fast Track 2WW Pelvic Ultrasound at York & Scarborough Teaching Hospitals
o MARKING IT AS Fast track/2WW PMB in the request
▪ History
▪ Risk factors for Endometrial Cancer
▪ BMI
▪ Examination findings
▪ Past Medical History
▪ Drug History
▪ WHO Performance status
- NICE guidance recommends referring ALL women with PMB >55 years old
- NICE guidance recommends to consider referring women with who are less than 55 years old taking into consideration risk factors for EC, recurrent episodes of PMB etc.
- NICE guidance recommends to consider direct access Pelvic Ultrasound for women aged > 55 years with:
i. Unexplained symptoms of vaginal discharge who:
1. Are presenting for first time OR have Thrombocytosis OR report haematuria.
ii. Visible haematuria and:
1. Low Haemoglobin OR Thrombocytosis OR High Blood Glucose
Secondary Care Management of patients referred on Fast Track/2WW PMB Pathway - see 'Referral Criteria/Information' section
Endometrial Assessment in Secondary Care
Outpatient Hysteroscopy Clinic
- Appointment for OP hysteroscopy should aim to have occurred by day 12 of pathway
- Written information about Out-patient hysteroscopy should be sent out to the patient with the appointment confirmation letter.
- All women should have a full medical history taken and BMI recorded. Written consent should be obtained in the clinic.
- If an out patient hysteroscopy is not possible or if the patient declines then an urgent daycase Hysteroscopy under general anaesthetic should be booked aiming for no later than day 18 of the pathway. If the patient has multiple medical co-morbidities then a consultant opinion (and possibly anaesthetic opinion) should be sought, a risk assessment of the risk of cancer vs. risk of anaesthetic is required. Also, a holistic assessment of patient if performance status is poor
- If out-patient hysteroscopy is possible, the findings should be clearly documented. The possible cause for bleeding should be recorded.
- Biopsy should be taken in all patients. The woman can be diagnosed as not cancer and removed from the fast track pathway only with normal histology.
- If a polyp is encountered and “see and treat” removal is not possible, a biopsy should be taken from the polyp. The patient should remain of the Fast Track pathway until the polyp has been removed and the histology result is known.
- It should be discussed with the patients how they would like to be informed of their results. If abnormal this would usually be a choice between a telephone call, ad hoc visit to the Women’s unit or an urgent clinic appointment. Those with normal results would usually receive a letter. Their decision should be documented in the notes.
- Patients should be informed as soon as possible via their chosen route. If abnormal then letters to the patient confirming diagnosis and possible further treatments should be sent urgently once they have received their diagnosis. This should include contact details of the clinical nurse specialist and patients also informed that they will be contacted directly by them to discuss their holistic needs and follow-up arrangements.
- A copy of the patient letter should be sent to the GP informing them the patient has received a diagnosis of cancer.
- There is no need to wait for the MDT confirmation if histology is endometrial adenocarcinoma, atypical hyperplasia, serous or clear cell adenocarcinoma plus carcinosarcomas.
- A Fast Track MRI or CT scan should be booked (see below) and U+E’s checked if not done recently.
|
Pathology |
Imaging required for radiological staging |
|
Atypical Hyperplasia |
Nil |
|
Endometrioid Adenocarcinoma (G1-G2) |
MRI Pelvis |
|
Endometrioid Adenocarcinoma (G3 |
CT Thorax Abdomen Pelvis |
|
Serous/Papillary/clear cell adenocarcinoma Carcinosarcoma |
CT Thorax Abdomen Pelvis |
- The woman needs to be discussed at the next MDT meeting even if MRI or CT not yet available as a plan or care can be made.
- Once a treatment plan is made by the MDT, the woman needs to be informed by letter or telephone call.
- Subsequent management would be directed by the MDT and would follow:
- YORK PATIENTS: the West Yorkshire and Harrogate Cancer Alliance Gynaecological Cancer guidelines. See for further information: https://www.wypartnership.co.uk/application/files/2215/1842/8918/WYH_Gynaeco logy_Guidelines_v4.2_September_2017.pdf
The guidelines for the management of gynaecological cancers in the Humber and North Yorkshire region are based on national standards and best practices set by the National Institute for Health and Care Excellence (NICE) and the British Gynaecological Cancer Society (BGCS), implemented via the Humber and North Yorkshire Cancer Alliance.
- SCARBOROUGH PATIENTS: the Hull University Teaching Hospital NHS Trust central MDT guidance
- YORK PATIENTS: the West Yorkshire and Harrogate Cancer Alliance Gynaecological Cancer guidelines. See for further information: https://www.wypartnership.co.uk/application/files/2215/1842/8918/WYH_Gynaeco logy_Guidelines_v4.2_September_2017.pdf
Special situations:(a) TAMOXIFEN
(b) INTRAUTERINE DEVICE (IUD)
(c) RECURRENT PMB
(d) INCIDENTAL INCREASED ENDOMETRIAL THICKNESS IN ASYMPTOMATIC WOMEN
(e) FLUID IN CAVITY BUT NORMAL ENDOMETRIAL THICKNESS
|
Unscheduled bleeding on HRT pathway
This pathway is for persons taking HRT experiencing unscheduled bleeding. It should be read alongside the BMS guidance for unscheduled bleeding on HRT.
Introduction
Unscheduled bleeding on Hormone Replacement Therapy (HRT) is defined as bleeding which occurs after initiating or changing an HRT preparation which should be bleed free (continuous combined HRT, ccHRT) or which occurs in addition to the scheduled monthly withdrawal bleed in persons taking sequential preparations (sHRT).
It is common to get unscheduled bleeding within the first 6 months of initiating HRT, or within 3 months of a dose change or preparation change in those already established on HRT. Up to 38% in sHRT and 41% in ccHRT users.
Assessment of women with unscheduled bleeding on HRT
Clinical assessment should start with detailed history, examination and identification of risk factors for cancer (see Risk factor table in section 1).
History
- LMP, withdrawal bleed (before and during HRT)
- Bleeding pattern before starting HRT
- Pelvic pain/deep dyspareunia
- Discharge
- Vulvo-vaginal and urinary symptoms
- Bleeding pattern
- Number episodes/month
- Type: spotting, period like, flooding
- Duration
- Regularity or timing in cycle (eg sHRT)
- Precipitating factors-wiping, post-coital
- HRT use:
- Duration since initiation or change in HRT preparation
- Current preparation-including type, route & dose of oestrogen and progestogen
- Type of progestogen, total days in month taken o Levonorgestrel IUD (52mg LN IUD)-type, date of insertion, thread checks, confirmation of correct situs
- Adherence to oestrogen and progesterone regime
- Prior preparations and adverse effects
- Application:
- Where applied o If patch sticking well, irritation?
- Other sources of oestrogen (herbal/bioidentical)
- Additional contraceptive use
- Drug interactions-anti-epileptics, anti-fungals, COVID vaccination, St Johns Wort
- Malabsorption syndromes
- Endometrial cancer risk factors: genetic risks (Lynch or Cowden Syndrome), BMI>30, PCOS, Diabetes
Examination and initial investigations:
- Abdominal-assess for fibroids, ovarian mass, pain
- Vulvo-vaginal-assess for atrophy, dermatoses, mass, ulceration, prolapse
- Cervical appearance-mass, polyp, ectropion with contact bleeding, visible LNG IUD threads
- Genital tract swabs
- Cervical screening if overdue
- Pregnancy test (if appropriate)
- BMI
Assess risk factors for Endometrial cancer:
- See major and minor risk factors for endometrial cancer (see 'red flags' section)
Assessment of HRT doses
Risk of endometrial cancer with HRT use is associated with the oestrogen dose and the proportionate dose/type of progestogen used for endometrial protection.
Oestrogen doses:
|
|
Ultra low dose |
Low dose |
Standard dose |
Moderate dose |
High dose |
|
Oestrogel |
½ pump |
1 pump |
2 pumps |
3 pumps |
4 pumps |
|
Sandrena |
0.25 mg |
0.5mg |
1mg |
1.5-2mg |
3mg* |
|
Lenzetto spray |
1 spray |
2 sprays |
3 sprays |
4-5 sprays* |
6 sprays* |
|
Patch |
12.5 microgram |
25mcg |
50mcg |
75mcg |
100mcg |
|
Oral Oestradiol |
0.5mg |
1mg |
2mg |
3mg* |
4mg* |
*Off license use
Recommended progestogen dose per licensed oestrogen dose in the baseline population
|
Oestrogen dose |
Micronized Progesterone (mg) |
Medroxyprogesterone (mg) |
Norethisterone (mg) |
LNG IUD 52mg |
|||
|
Continuous |
Sequential |
Continuous |
Sequential |
Continuous |
Sequential |
For up to 5yrs of use |
|
|
Ultra low/low |
100 |
200 |
2.5 |
10 |
5^ |
5 |
|
|
Standard |
100 |
200 |
2.5-5 |
10 |
5 |
5 |
|
|
Moderate |
100 |
200 |
5 |
10 |
5 |
5 |
|
|
High |
200* |
300* |
10* |
20* |
5 |
5 |
|
*There is limited evidence on optimal dose and safety for endometrial protection for high dose oestrogen regimes
^1mg is sufficient but 5mg is lowest stand alone dose in UK (off license use of 3x noriday (1.05mg) could be use if 5mg NET not tolerated
When to investigate unscheduled bleeding on HRT - see 'Referral Criteria/Information' section
Recommendations for management according to endometrial histology
If patients have been assessed in gynaecology clinic and had an endometrial biopsy, their onward management is determined by the biopsy result and nature of endometrial assessment.
PIPELLE BIOPSY:
- If blind pipelle endometrial biopsy is reported as normal, offer reassurance and discuss adjustments in the progestogen/HRT regimen for 3 months. If unscheduled bleeding persists after this interval, or becomes heavy / prolonged, offer hysteroscopic assessment on an urgent pathway (within six weeks).
- A hysteroscopy should be offered, on an urgent pathway (within six weeks), in the presence of a thickened ET on TVS and a blind biopsy which is reported as an ‘insufficient sample’.
- If proliferative endometrium is reported on blind biopsy and there are risk factors for endometrial cancer (1 major or 2 minor) and the preparation used is ccHRT, offer hysteroscopy.
HYSTEROSCOPY AND BIOPSY:
- In the presence of a normal biopsy and hysteroscopy, discuss adjustments in the progestogen/HRT regimen and provide reassurance for six months. If unscheduled bleeding persists after this interval, or becomes heavy / prolonged, offer a repeat TV Ultrasound on an urgent pathway. If the ultrasound demonstrates increased endometrial thickness (sHRT ET>7mm, ccHRT > 4mm), refer Fast Track Gynaecology PMB (Urgent suspected Cancer Pathway) •
HYPERPLASIA OR CANCER:
- If hyperplasia with atypia or endometrial cancer is reported, advise weaning off HRT, discuss non-hormonal alternatives and refer to gynaecology oncology on an USCP.
Adjusting HRT to reduce unscheduled bleeding episodes
Interventions can reduce unscheduled bleeding episodes, see table for recommendations for reducing and managing unscheduled bleeding on HRT:
|
Problem |
Potential adjustments |
|
General Principles |
|
|
Poor compliance of noncombined methods |
|
|
Submucosal/Intramural fibroids |
|
|
BMI ≥ 30 |
|
|
Perimenopausal and unscheduled bleeding with sHRT |
|
|
Unscheduled bleeding with ccHRT |
|
Special situations:A-Incomplete assessment of Endometrium
|
Referral Criteria/Information
If endometrial cancer is suspected:
- Make referral to Fast Track Gynaecology 2WW PMB (Urgent suspected Cancer Pathway)
AND
- Make referral/request for Fast Track 2WW Pelvic Ultrasound at York & Scarborough Teaching Hospitals
- MARKING IT AS Fast track/2WW PMB in the request
▪ History
▪ Risk factors for Endometrial Cancer
▪ BMI
▪ Examination findings
▪ Past Medical History
▪ Drug History
▪ WHO Performance status
Secondary Care Management of patients referred on Fast Track/2WW PMB Pathway
- Referral for Fast Track Gynaecology PMB (Urgent suspected Cancer Pathway) is triaged via NOTIFY
- PMB referrals are triaged to “PMBUSSREVIEW Clinic”
- Referrals made by non-standard pathway are assessed and if already has had USS then triaged into correct clinic pathway (see below) 2
- There will be a York site and a separate Scarborough site “PMBUSSREVIEW Clinic” depending on the catchment locality of the patient and locality of referring primary care practice
- Gynaecology Admin Team map “PMBUSSREVIEW Clinic” appointment to the day of FT Pelvic USS that primary care requested at point of referral (aiming for no later than Day 9 of the Pathway).
- If USS has not been requested alert gynae oncology triage team:
i. Who will request USS & write to referring clinician reminding them of their requirement to request USS at point of referral
- If USS has not been requested alert gynae oncology triage team:
- Gynae Oncology triage team review the “PMBUSSREVIEW Clinic” each day
- As failsafe the preceding 3 “PMBUSSREVIEW Clinic” sessions are reviewed to ensure all patients USS have been reviewed and clinic outcomes completed.
- The outcomes of the “PMBUSSREVIEW Clinic” are:
- Outcome 1.
i. Endometrial Thickness < 4mm, rest of USS is normal.
ii. Stop FDS clock, discharge patient, Standard letter 1. - Outcome 2.
i. USS result abnormal requiring endometrial assessment
• ET≥4mm • Recurrent PMB
• Tamoxifen use
• ET unmeasurable
• Irregular Endometrium
• Suspected polyp or focal pathology
ii. Refer to Fast Track Out-patient Hysteroscopy Clinic, Standard letter 2 - Outcome 3.
i. USS= Endometrium normal but suspicious adnexal mass
ii. Request ca125 +/- CT/MRI as per adnexal mass pathway
iii. Refer to Fast Gynae outpatients clinic, Standard letter 3. - Outcome 4.
i. USS=endometrium normal, but other benign finding
ii. Stop FDS clock, refer to Urgent New Patient Gynae Clinic, Standard letter 4.
- Outcome 1.
When to investigate unscheduled bleeding on HRT
A. WHEN TO REFER FAST TRACK GYNAECOLOGY PMB:
Women with unscheduled bleeding on HRT, irrespective of duration of use, with: 1 major or 3 minor risk factors for Endometrial Cancer:
- Make referral to Fast Track Gynaecology 2WW PMB (Urgent suspected Cancer Pathway)
AND - Make referral/request for Fast Track Pelvic Ultrasound at York & Scarborough Teaching Hospitals
- MARKING IT AS Fast track/2WW PMB in the request
▪ History
▪ Risk factors for Endometrial Cancer
▪ BMI
▪ Examination findings
▪ HRT information: dose, type, duration, when and whether or not stopped prior to ultrasound, compliance
▪ Past Medical History
▪ Drug History
▪ WHO Performance status
B. WHEN TO REFER FOR AN URGENT 6 WEEK ULTRASOUND PELVIS:
- 2 minor risk factors for endometrial cancer
- Within any time frame of starting ccHRT/sHRT:
- Prolonged withdrawal bleeds > 7days
- Heavy bleeding-flooding/clots
- Persistent bleeding-most days for 4 weeks or more
- More than 3 months after a change in dose/preparation
- More than 6 months after starting HRT and:
- Reports bleeding with ccHRT
- Develops unscheduled bleeding on sHRT having previously had light and regular withdrawal bleeds
What to do with the results of the USS:
- Endometrial thickness: if >4mm ccHRT or >7mm sHRT referral to Fast track Gynaecology PMB
- If endometrium within normal limits offer HRT adjustments
- If the bleeding persists 6 months after these changes or increases in intensity/frequency recommend:
- Make referral to Fast Track Gynaecology PMB (Urgent suspected Cancer Pathway)
AND - Make referral/request for Fast Track Pelvic Ultrasound at York & Scarborough Teaching Hospitals
C. WHEN TO MANAGE CONSERVATIVELY AND OFFER ADJUSTMENTS TO HRT
- Can be offered to women with no risk factors for endometrial cancer
- Unscheduled bleeding within 6 months of initiation of ccHRT or sHRT
- Unscheduled bleeding continuing 3 months after a change in HRT dose or preparation
- If bleeding occurring three months after this change offer adjustments for a total of six months
- If unscheduled bleeding continues after 6 months of adjustments, discuss:
- Urgent (6 week) USS OR
- Weaning off HRT and consideration of non-hormonal alternatives (to avoid invasive investigations)
- If bleeding ceases at a 4 week follow up, and continuing without HRT is acceptable, no further investigation is required
- If preference to restart HRT then adjust HRT for 6 months, if bleeding continues during HRT adjustments recommend Urgent USS Pelvis within 6 weeks
- See above for guidance regarding results of USS
Additional Resources & Reference
Associated Policies
Specialties
Places covered by
- North Yorkshire
- Vale of York
Hospital Trusts
-
York and Scarborough Teaching Hospitals